Human African Trypanosomiasis (HAT), also known as sleeping sickness, remains a threat to communities in remote regions of Africa. Caused by parasites and transmitted by infected tsetse flies, it is fatal if left untreated. It causes neuropsychiatric symptoms, including aggressiveness, psychosis, a debilitating disruption of sleep patterns, and ultimately death. There are two forms of sleeping sickness: T.b, gambiense, the more common variant, and T.b. rhodesiense, an acute form of this parasitic disease.
Foundation S is dedicated to eliminating sleeping sickness by 2030 through innovative treatments and unique public and private partnerships.

Elimination is on the Horizon
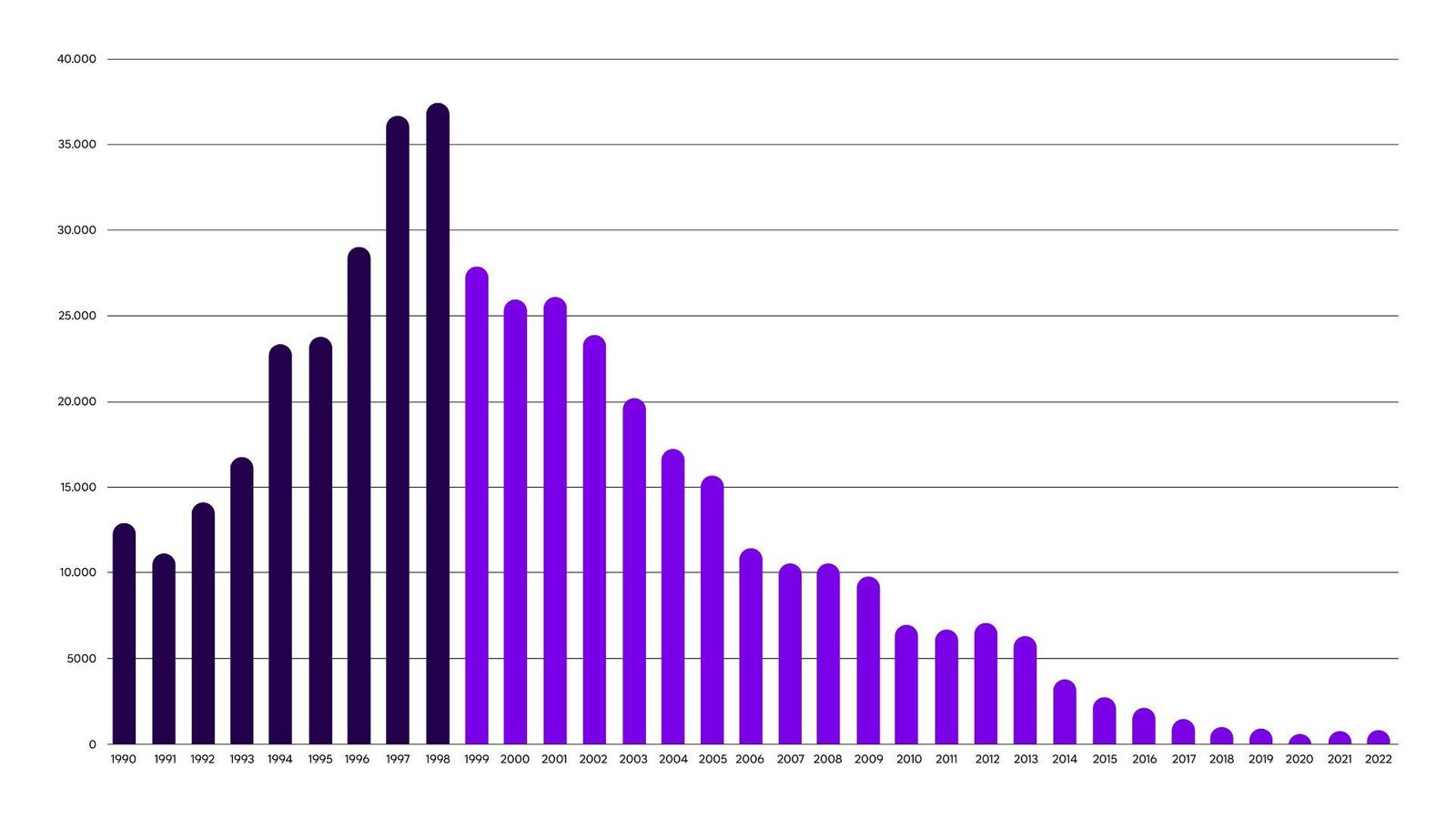
While cases have dropped dramatically in recent years thanks to better monitoring and wider treatment options, complex administration methods remain a challenge in underserved populations.
Through long-standing partnerships with the World Health Organization (WHO) and non-profit organization DNDi (Drugs for Neglected Diseases Initiative), we have been on a journey to revolutionize treatment options, and donate these life-saving drugs to patients in need. We have contributed to a staggering a 98% reduction in cases since 2001 and secured access to treatment for 200,000 patients.
Recently, Guinea became the 9th country to eliminate the disease, marking a significant milestone in the fight against sleeping sickness. We now stand on the verge of a new game-changing drug, that will be critical in driving further progress towards elimination.
We must stay mobilized and maintain our momentum to complete this mission, or we risk losing decades of progress. Together, we can build on the progress made and secure a future free from another deadly NTD.
Our impact
98%
reduction in cases since 2001
What we support
Alongside the DNDi, we have been on a remarkable scientific journey – developing safer and more effective treatment options for patients impacted by this life-threatening disease. This includes the development of Fexinidazole, the first multi-dose, all-oral treatment, which revolutionized treatment outcomes and simplified delivery.
Now, we are advancing Acoziborole - a first-of-its-kind, single-dose oral treatment poised to enable a groundbreaking test-and-treat approach. If approved, it could be the game-changing treatment we need to accelerate elimination of this awful disease.
Related Content

Eliminating Sleeping Sickness Has Never Been Closer

EMA Gives Positive Opinion to Fexinidazole Winthrop
as first oral treatment of acute form of sleeping sickness (rhodesiense) found in East and Southern Africa

Acoziborole
Investigational single-dose oral treatment raises hope for elimination of sleeping sickness in Africa.